Category: Insight
Maxstim’s role in global food security
Innovative solutions are needed to ensure that there will be adequate global food security and food supply in the future.
Previously we’ve discussed some of the issues that are already affecting the many processes within our food system, including a changing climate, a growing population and unstable economies. Read our discussion here.
The global food security challenges we’re facing
Global food security is facing huge challenges. Currently, in 2022, about 829 million people are undernourished.
Food security, defined by the United Nations’ Committee of World Food Security, means that all people, at all times, have physical, social and economic access to sufficient, safe and nutritious food that meets their food preferences and dietary needs for an active and healthy lifestyle. Furthermore, the individuals involved in this food production are able to earn adequate wages.
Maxstim for Grapes increases the growth, yield and quality of dessert grapevines
Laboratory, glasshouse and commercial field trials have demonstrated that the Maxstim range of biostimulants will increase growth, enhance yields and improve quality in a wide range of agricultural and horticultural crops. The Maxstim team are continually extending their knowledge on how best to support growers in their use of biostimulants by conducting research and efficacy trials on new crops and crop varieties.
Biostimulant data: What crop categories and trials are used?
EBIC suggestions on underlying principles to justify a biostimulant claim:
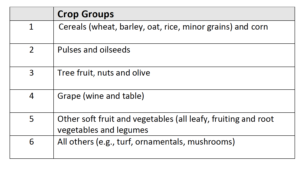
Crop categories and Number of Trials
Six groups of crops have been identified for confirming biostimulant efficacy:
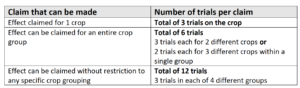
The number of trials required for a product depends on the crop range to which the claim is being made
Trials must be conducted by qualified personnel who will record, document, and archive the trial study plan, the results, the final report and all the supporting raw data.
Data from GEP/GLP-certified facilities can be considered credible, even if they belong to the manufacturer. Nonetheless, it is desirable that manufacturers can demonstrate that at least some of the research was conducted with impartial and competent third parties.
As much as possible, trials to support product claims should be conducted with an independent and competent partner, such as one of the following:
- National research agencies and extension officers;
- Institutes (including but not limited to universities and other institutes of higher learning and private research stations)
- Researchers with published research in agriculture and agronomy, and
- Certified private research centres (GLP/GEP or GLP/GEP equivalent conditions).
Trial conditions
The relevant conditions of the plot and crop should be adequately described, for example:
- For an annual crop, sowing or planting date and density, row spacing;
- For a perennial crop, arrangement and spacing in rows or as single plants, pruning or training system, rootstock, canopy height, plant width, age, whether in production;
- For a glasshouse crop, arrangement within compartments, on benches, in soil-less culture, etc.;
- The cultivation practices of the crop could be described, such as tillage, fertilizer and irrigation regimes, and any other additional inputs;
- Information should be given on whether the crop was growing normally or was under stress at the time(s) of treatment [e.g., drought, frost, wind or effects of other overall chemical treatments, and/or effects of other pests (including diseases and weeds)];
- For a soil-applied product, the temperatures at the root zone level in topsoil should be recorded during at least the first month of the trial at 2-h intervals), and
- Soil characteristics should be described, i.e. pH, the percentage of sand, clay, silt, organic matter
Design and lay-out of trials
- The design and lay-out of the plots should be described, preferably with a plan, the number, size and shape of plots, whether defined by plot dimensions on the ground or a certain lay-out of plants.
- The type of experimental design should be indicated.
- The arrangements made for the untreated control (included, imbricated, and excluded) should be precisely indicated, together with details on any other control treatments.
- Completely randomized blocks should be assured, while maintaining a scientific design that avoids any interference of experimental conditions between plots (for example, with regards to drought stress mitigation trials, the well-watered condition will have to be set up as a band reference beside the trial.
- Enough replicates should be assured to obtain 12 degrees of freedom in the trial, so that a consistent difference between treated and untreated crops can be demonstrated.
Control data
The control data set can be from a completely untreated plot or an “omission” plot i.e., the treatment regimen is the same with the exception of the biostimulant, which is absent from the “omission” plot.
Where possible, control groups selected for an improved nutrient uptake claim should include:
- Untreated
- The following additional control groups, when a biostimulant is included in a “support” nutrient-containing formulation:
1. The support formulation alone, if the support provides nutrient elements;
2. The biostimulant formulation alone (without the nutrient elements).
- Abiotic stress resistance claims should include the following control groups:
1. Stress condition object(s);
2. No-stress condition object(s);
There should be some attempt to characterize the applied stress level.
Application of treatments
Information should be provided on the formulation, application method, concentration and amounts of the test product as well as climatic and soil data.
Mode of application:
The information provided should be sufficient to establish that good agricultural practice is being followed, for example:
- The application method and equipment used
- Any significant deviations from the intended dosage
- The operating conditions, insofar as they may affect claims (e.g., for sprays, pressure, nozzle type, spray quality and speed of travel of sprayer)
- The number of applications
- The date of each application (including year, preferably by dd-mm-yyyy)
- The growth stage of the crop at the time of each application
- The doses used (cc-g/hL or L-Kg/ha), and the spray volumes (L/ha).
Mode of assessment:
- Type, time and frequency of assessment
- The type and date of each assessment
- The methods used should be described. Any assessment scales used should be specified.
- Direct effects on the crop. The presence or absence of phytotoxic effects should be noted for each plot, with an accurate description of any symptoms, for example: modifications in the development cycle, thinning, modifications in colour, necrosis, deformations, effects on the quantity and quality of the yield.
- Yield and quality should, when specified, be recorded, taking careful note of the specific parameters required in each crop.
The trial series report
The trial report should include:
- The aim of the trial series
- The list of test and reference products, with doses and application times of frequencies
- The assessment methods
- Results including statistical analysis if any were conducted.
How biostimulants can help solve the fertiliser problem
We’ve previously shared with you our insight on the effect of biostimulants on plants in the context of the global over usage of fertilisers.
We’ve looked at the need for biostimulants as a way to enable plants to better use the nutrients available to them, therefore allowing producers to use less fertilisers, the effect of biostimulants on plant roots, how they can reduce nitrogen requirement, and most recently their influence on soil structure and the microbial activity in soil.
In this final article we round up our conclusions on the effect and opportunity of biostimulants to help reduce global fertiliser consumption. And share the insights we’ve evidenced using Maxstim biostimulants in trials.
Can plant biostimulants improve microbial activity in soil?
There are a number of strategies where biostimulants can help the fertiliser problem.
Our previous articles gave insight to the fertiliser problem we are facing globally and demonstrated the nitrogen cycle. We introduced the need for biostimulants as a way to enable plants to better use the nutrients available to them, therefore allowing producers to use less fertilisers , the effect of biostimulants on plant roots and how they can reduce the nitrogen requirement.
Can plant biostimulants improve soil structure?
There are a number of strategies where biostimulants can help the fertiliser problem.
Our previous articles gave insight to the fertiliser problem we are facing globally and demonstrated the nitrogen cycle. We introduced the need for biostimulants as a way to enable plants to better use the nutrients available to them, therefore allowing producers to use less fertilisers , the effect of biostimulants on plant roots and how they can reduce the nitrogen requirement.
Can plant biostimulants promote nitrogen reduction?
The influence of biostimulants on plant roots
There are a number of strategies whereby biostimulants and plant nutrients can help ‘the fertiliser problem’.
Our previous article gave some insight into the fertiliser problem we are facing globally and demonstrated the significance of the nitrogen cycle. We introduced the value of biostimulants as a way to enable plants to better use the nutrients available to them, therefore allowing producers to use fewer fertilisers. Read it here.
So what are the specific strategies where biostimulants can make a difference?
The following article shares insight specifically on the influence of biostimulants on the root system in plants.
Improve plant nutrient (especially nitrogen and phosphorous) uptake by altering plant morphological characteristics (Halpern et al., 2015) e.g. Changes to root nutrients’ parameters (e.g. structure and morphology – we have evidence that Maxstim formulations can achieve this)
Plant root structure can respond to the application or availability of biostimulants by changing their root growth. Changes in root architecture and surface area can enable plants to explore a larger volume of soil and capture nutrients more effectively. Consequently, soil containing a lower level of nutrient can deliver the same amount of nutrient to the crop if the plant roots are growing through and accessing a larger soil volume.
Imagine being able to have 20% more crop for the same amount, or less, of root fertiliser.
Root morphology: It is known that root morphology is important for acquiring nutrients with low mobility in the soil (Nye & Tinker 1977). However, for more mobile nutrients, e.g. NO3 – and NH4 +, root morphology is often considered of lesser importance. Forde and Lea (2007) reported a significant effect of the amino acid glutamate, even at very low concentrations, in changing root architecture by inhibiting primary root growth and increasing root branching near the root apex. The increase in root branching induced by glutamate application can enhance the density of the root system improving the plant’s ability to explore the soil volume and to uptake nutrients and water.
Biostimulant effects of protein hydrolysates (which contain amino acids) can also result from changes in the microbial community of the rhizosphere. For example, Luziatelli et al. (2016) in a lettuce trial reported that the application of a plant derived- protein hydrolysates increased the population of bacterial species producing the auxin, 3-indoleacetic acid, and other auxin-like compounds. Changes in plant growth are often due to the presence of bioactive peptides inducing hormone-like activities e.g. auxin, gibberellins, and brassinosteroids, which involve complex interactions among phytohormones (Colla et al., 2015; Kim et al., 2019).
Root to shoot ratio: One way for a plant to access more root nutrients is to develop a larger root system. However, this strategy comes at a cost. Larger root systems may divert more carbon away from the shoots, limiting photosynthesis and a crop’s capacity to fix and store carbon in the harvested yield. Some studies indicate that the relationship between root size and yield at low N is not clear and may be negative (Gallais & Coque 2005), so developing larger roots may be a sub-optimal strategy for some crop plants. With high levels of nutrition, root to shoot ratios are generally lower and under these conditions, parameters such as pH and temperature may be more important for N uptake than root morphology. The root to shoot ratio also changes as the plant develops, with the general trend (at least with herbaceous plants) being towards a relatively smaller root system as the plant ages, independent of nutrient level.
Root length density: Root length density is the root length per volume of soil. Greater root length density (e.g. greater numbers of smaller diameter, as opposed to fewer large, roots) can improve nutrient acquisition by increasing the root surface area without requiring an increase in carbon allocation to the root (Marschner 1995). Accordingly, it has been suggested that increasing root length density may improve N acquisition in some crops.
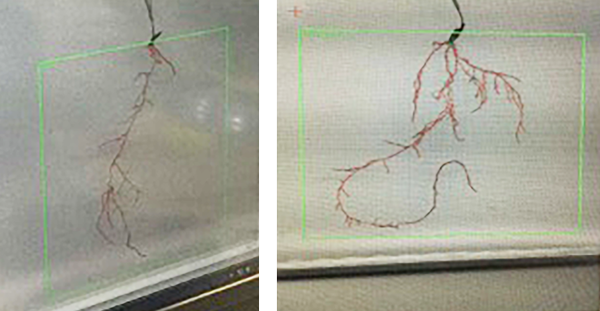
The image below shows the effects of Maxstim’s complex biostimulants on rye grass root – untreated vs treated.
Root vigour: Simply having a larger root to shoot ratio over the plant’s lifespan may not always be useful for increasing Nitrogen usage efficiency in some agricultural situations but the ability to develop a large root system early would seem to be of some benefit in certain conditions. Liao et al. (2004, 2006) found that, in sandy soils with high leaching potential, the ability of plants to rapidly explore soil and capture available NO3 – was important for optimising nitrogen uptake from the soil. These plants did not have a higher root to shoot ratio at final harvest, but their root development was more rapid than in less vigorously rooted plants.
Root proliferation in response to Nitrogen: While root growth relative to shoot growth is generally reduced when soil N levels are high, it is known that roots will proliferate in response to localized patches of high N (Drew et al., 1973; Drew, 1975; Drew & Saker, 1975; Laine et al., 1995). This would appear to be an evolutionary adaptation so that root allocation is not wasted in areas of the soil containing little N.
Deep roots v shallow roots: Deeper roots clearly have the potential to access more water and nutrient than shallow roots but root length densities are generally lower deeper in the soil profile (Barraclough 1986; Wiesler & Horst 1993, 1994). In porous soils where surface applied N is rapidly distributed deep within the soil horizon, plants which can develop a greater root mass further down the length of the root system would have an advantage in enhancing N uptake and reducing N leaching losses from the soil.
Root hairs: Root hairs can contribute 70–80% of the total root surface area and therefore play a critical role in nutrient uptake. These structures are important in increasing the surface area of roots through a larger effective root diameter with a relatively low investment in dry matter and allocated Carbon. It has been demonstrated that root hair number and density increase in response to nutrient stress. Exogenous application of amino acid has also been shown to have an effect on root morphology. Specifically, L-glutamate application to the root, inhibited primary-root growth and stimulated root branching (Walch-Lui et al., 2006). It also stimulated root-hair development close to the root tip (Walch-Lui et al., 2006). This effect was specific to L-glutamate, and did not occur in response to applications of 21 other amino acids, including D-glutamate.