The importance of data in assessing the efficacy of biostimulants
EBIC suggestions on underlying principles to justify a biostimulant claim:
Crop categories and Number of Trials
Six groups of crops have been identified for confirming biostimulant efficacy:
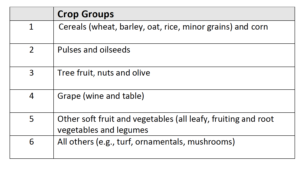
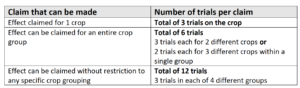
The number of trials required for a product depends on the crop range to which the claim is being made
Trials must be conducted by qualified personnel who will record, document, and archive the trial study plan, the results, the final report and all the supporting raw data.
Data from GEP/GLP-certified facilities can be considered credible, even if they belong to the manufacturer. Nonetheless, it is desirable that manufacturers can demonstrate that at least some of the research was conducted with impartial and competent third parties.
As much as possible, trials to support product claims should be conducted with an independent and competent partner, such as one of the following:
- National research agencies and extension officers;
- Institutes (including but not limited to universities and other institutes of higher learning and private research stations)
- Researchers with published research in agriculture and agronomy, and
- Certified private research centres (GLP/GEP or GLP/GEP equivalent conditions).
Trial conditions
The relevant conditions of the plot and crop should be adequately described, for example:
- For an annual crop, sowing or planting date and density, row spacing;
- For a perennial crop, arrangement and spacing in rows or as single plants, pruning or training system, rootstock, canopy height, plant width, age, whether in production;
- For a glasshouse crop, arrangement within compartments, on benches, in soil-less culture, etc.;
- The cultivation practices of the crop could be described, such as tillage, fertilizer and irrigation regimes, and any other additional inputs;
- Information should be given on whether the crop was growing normally or was under stress at the time(s) of treatment [e.g., drought, frost, wind or effects of other overall chemical treatments, and/or effects of other pests (including diseases and weeds)];
- For a soil-applied product, the temperatures at the root zone level in topsoil should be recorded during at least the first month of the trial at 2-h intervals), and
- Soil characteristics should be described, i.e. pH, the percentage of sand, clay, silt, organic matter
Design and lay-out of trials
- The design and lay-out of the plots should be described, preferably with a plan, the number, size and shape of plots, whether defined by plot dimensions on the ground or a certain lay-out of plants.
- The type of experimental design should be indicated.
- The arrangements made for the untreated control (included, imbricated, and excluded) should be precisely indicated, together with details on any other control treatments.
- Completely randomized blocks should be assured, while maintaining a scientific design that avoids any interference of experimental conditions between plots (for example, with regards to drought stress mitigation trials, the well-watered condition will have to be set up as a band reference beside the trial.
- Enough replicates should be assured to obtain 12 degrees of freedom in the trial, so that a consistent difference between treated and untreated crops can be demonstrated.
Control data
The control data set can be from a completely untreated plot or an “omission” plot i.e., the treatment regimen is the same with the exception of the biostimulant, which is absent from the “omission” plot.
Where possible, control groups selected for an improved nutrient uptake claim should include:
- Untreated
- The following additional control groups, when a biostimulant is included in a “support” nutrient-containing formulation:
1. The support formulation alone, if the support provides nutrient elements;
2. The biostimulant formulation alone (without the nutrient elements).
- Abiotic stress resistance claims should include the following control groups:
1. Stress condition object(s);
2. No-stress condition object(s);
There should be some attempt to characterize the applied stress level.
Application of treatments
Information should be provided on the formulation, application method, concentration and amounts of the test product as well as climatic and soil data.
Mode of application:
The information provided should be sufficient to establish that good agricultural practice is being followed, for example:
- The application method and equipment used
- Any significant deviations from the intended dosage
- The operating conditions, insofar as they may affect claims (e.g., for sprays, pressure, nozzle type, spray quality and speed of travel of sprayer)
- The number of applications
- The date of each application (including year, preferably by dd-mm-yyyy)
- The growth stage of the crop at the time of each application
- The doses used (cc-g/hL or L-Kg/ha), and the spray volumes (L/ha).
Mode of assessment:
- Type, time and frequency of assessment
- The type and date of each assessment
- The methods used should be described. Any assessment scales used should be specified.
- Direct effects on the crop. The presence or absence of phytotoxic effects should be noted for each plot, with an accurate description of any symptoms, for example: modifications in the development cycle, thinning, modifications in colour, necrosis, deformations, effects on the quantity and quality of the yield.
- Yield and quality should, when specified, be recorded, taking careful note of the specific parameters required in each crop.
The trial series report
The trial report should include:
- The aim of the trial series
- The list of test and reference products, with doses and application times of frequencies
- The assessment methods
- Results including statistical analysis if any were conducted.

