What data is required to show biostimulants are effective?
The importance of data in assessing the efficacy of biostimulants.
Type of information that can support a claim
Various types of data and empirical evidence can support a claim justification. While not strictly speaking hierarchical, it makes sense to begin with published peer reviewed scientific literature and existing data and then to complement that information as needed with new experimental data from controlled conditions and field trials
Data generated under controlled conditions (glasshouse, growth chambers, phenotyping, etc.) from outside the European Union should be admissible if the climatic conditions tested could conceivably apply within the EU and:
- if it is from a manufacturer’s own GEP/GLP-certified facilities.
- if the independent research partner (contract facility, university, etc.) that generated the data is considered reputable, or
- if the manufacturer can otherwise demonstrate that the quality of the methodology and the data obtained are substantially equivalent to what would be achieved by a GEP/GLP facility.
Use of Published Literature and Existing Data
Peer-reviewed scientific literature can support a claim.
Literature can be used to describe the mode of action of the product, the biology of the microorganisms used, or any preliminary studies described in relevant published papers supporting the basis of the proposed claim.
Scientific literature can be used to support a claim if it is of acceptable quality, (e.g. as per the criteria outlined in Klimisch et al. (1997). At the same time, the synergistic or emergent effects that result from the combination of substances within a product mean that it is unlikely literature alone will be enough to fully justify a claim.
Experimental Data
Biostimulant claims can be supported by experimental data generated under controlled conditions (laboratory, greenhouse, growth chamber, phenotyping, etc.) and/or in the field (field trials).
Additional data from small-scale laboratory and growth chamber studies will often form a vital component of the overall claim justification package provided.
If field data are used, at least some EU data should be included.
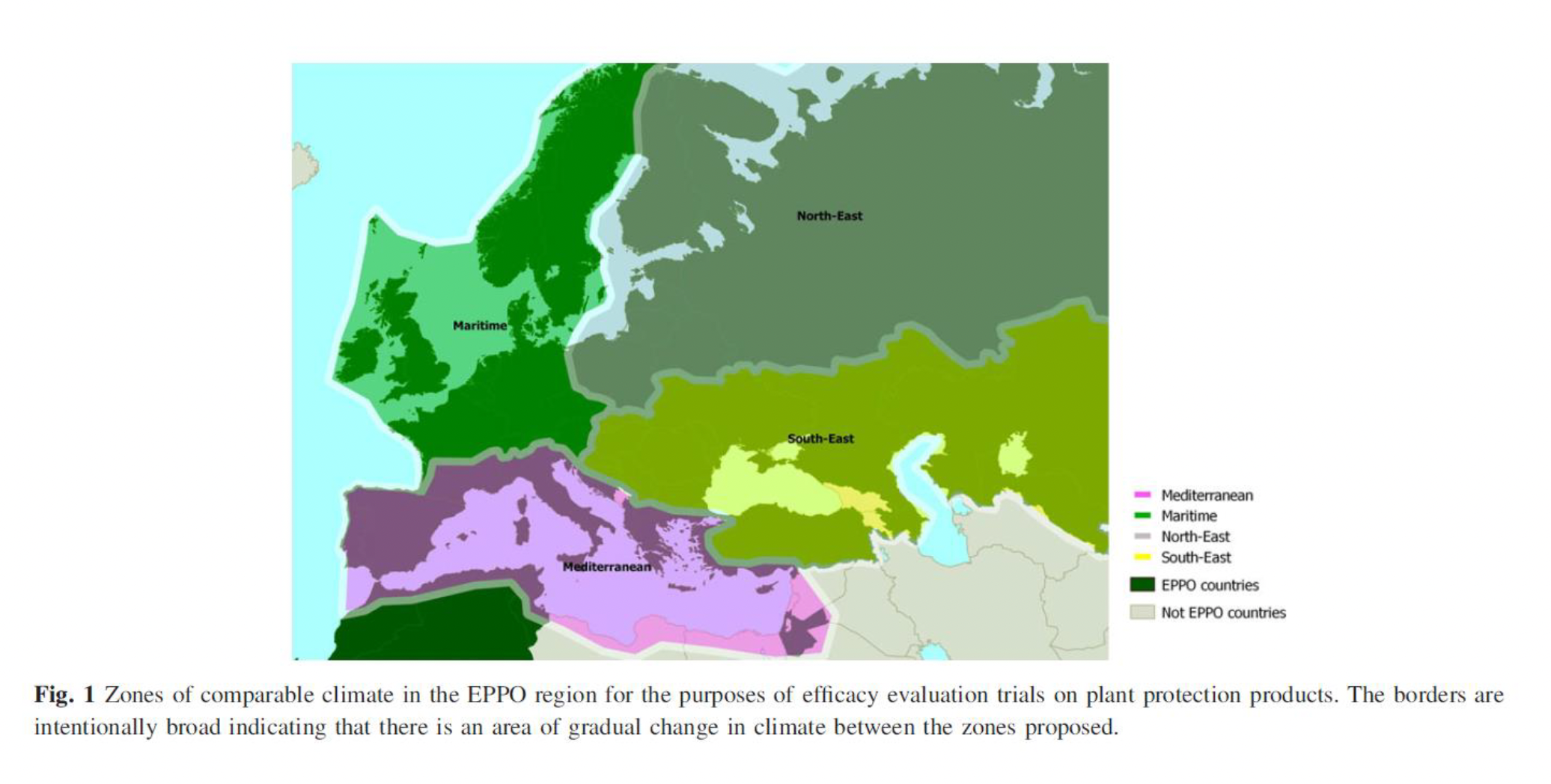
Field data from outside the EU may support EU data if both are generated under similar geo-climatic conditions (and those correspond to the intended context for product use). Guidelines exist for determining the comparability of geo-climatic conditions (European and Mediterranean Plant Protection Organization [EPPO], 2014).
Field trials provide essential information about biostimulant effects under real-world conditions. However, for some claims, such as salt stress or cold stress, it is difficult to artificially create the appropriate field conditions. In such cases, the focus of field trials would be more on the “holistic” benefits of the biostimulant in terms of yield/quality, while a specific biostimulant claim related to its mode of action could be demonstrated in controlled conditions.
The net agricultural benefit after considering both the positive and negative effects of the biostimulant should be large enough to justify its use. The benefit from the use of a product should be appropriate to the agronomic setting in which the product will be used. A low level of benefit may be acceptable in some situations,
for example:
- when a product will be used as a component of an Integrated Crop Management (ICM) program, or
- in specialized situations, such as organic farming, or where the product makes it possible to maintain the same level of yield or quality while decreasing nutrient applications.
General Guidelines for Trials/Assays of Biostimulants
Trial study plan
The trial study plan should cover the following topics:
- The aim of the trial series
- Statistical analysis and trial design
- Trial conditions
- Design and lay-out of trials
- Control data
- Application of treatments
- Mode of assessment.
Objective of the trial and basic information on the trial site.
The objective of the trial should be specified, including:
- The biostimulant effect(s) to be demonstrated
- The inclusion of other variables in the trial (dose rates, application conditions)
- Whether the trial is for evaluation of a claim or another purpose (germination test, quality of the harvested product, effects on succeeding crops, etc.).
The following basic information should be provided on the trial site:
- Full address and geographical coordinates, if possible
- Crop and cultivar
- Any useful details on the site (e.g., exposure and slope).
Statistical analysis and trial design.
The placing of a CE marked biostimulant on the market should never be considered to guarantee effectiveness under all conditions, as many factors may influence the performance of a biostimulant in the field. Many additional factors are relevant to biostimulant products when determining acceptable, beneficial, levels of action.
These can include:
- Offering an approach compatible with ICM systems and/or organic farming
- Greater compatibility with cultivation practices
- Mitigating undesirable effects (on human beings, beneficial organisms, non-target organisms, other crops etc.) of the alternative production system
In such cases, Maxstim should ensure that users can be provided with accurate information on the likely performance of the product and advice on how best to use the product so that it will perform as effectively and consistently as possible.
Under controlled conditions (greenhouse or laboratory), the level of confidence compared to an untreated control can be set at 90% probability (minimum for agronomic production trials in controlled conditions), given the nature of biostimulant effects and their inherent variability (physiology and biology sensitive to pedo-climatic conditions, local microbiome biodiversity and crop genetics).
Plant genotypes can influence the efficacy of biostimulants.
A minimum of three field trials in the EU should be performed to demonstrate the desired biostimulant claim. The observation of consistent “agronomically” positive data trends (i.e., not necessarily statistically significant) compared to untreated plots in field trials could be considered sufficient to justify a biostimulant claim (e.g., a meta-analysis of data could be used)
Trials do not necessarily need to be performed over multiple seasons if there are enough trials in one season carried out in the different geo-climatic conditions relevant to the agronomic conditions for which the product will be sold.
Trial design should permit discrimination between treated and untreated plots. There should be enough replicates to reduce the variability of the data and increase the chance to identify any consistently differences with the untreated plots.
Where a biostimulant’s performance is affected by temperature, soil type, crop, or other parameters, the trial design, execution, and subsequent user recommendations should
take these factors into account. Furthermore, some claims may be better tested under controlled conditions (e.g., abiotic stresses may be difficult to induce in the field).
When designed accordingly, multiple claims may be demonstrated in a single trial.
Untreated plot trial results in terms of yield and quality should reflect normal agronomic expectations for local production.
The study plan should specify what is assessed, how it is assessed, when, and why.
It is recommended to evaluate at least one measurable indicator related to a benefit perceived by the farmer (i.e., impact on yield and/or quality). For example, nodulation is an early measurable parameter, but it is not sufficient on its own to confirm an effect on yield and/or quality of the crop. If an indicator of biostimulation is not assessed in the trials designed to justify a claim, then this indicator cannot be claimed on the label. For example, a claim to increase root growth is measured and demonstrated in an experimental trial, but the crop yield increase is not quantified in the trial. Consequently, the label can claim “increased root growth” but cannot claim “improved yield” because an increase in yield was not quantified in the claims justification data.
Phytotoxicity
Phytotoxicity should be verified during trials. If no evidence of phytotoxicity is observed, then there is no need to conduct additional phytotoxicity trials. However, if signs of phytotoxicity are observed, then phytotoxicity data are needed.

